Determination Of Antioxidants And Phenolic Constituents By Liquid Chromatography With Multi-channel Electrochemical Detection
Zhu Yongxin, Zhou J, Long H, Tian F, Janle E, Kissinger, P.
Bioanalytical Systems, Inc., 2701 Kent Avenue, West Lafayette, IN, 47906, USA
Introduction
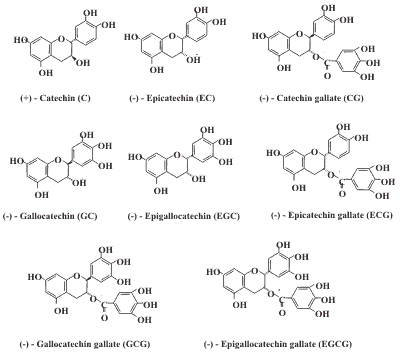
Antioxidants and phenolic constituents are widely present in botanical products, such as green tea, soy bean, grape, grape juice, wine and herbs such as Ginseng, Ginkgo biloba, St. John'sWort. Compounds which are antioxidants by virtue of their ability to act as reductants in solution tend to be easily oxidized (loss of electrons). Sensitive and selective liquid chromatography methods with multi-channel electrochemical detection have been developed for determining many of these compounds in extracts and in biological samples.
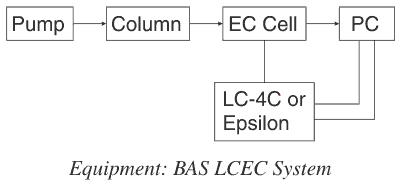
In this technique, the components of a complex mixture such as found in most botanical products are first separated by HPLC. The separated components can then be analyzed at up to four different electrodes set at four different voltages simultaneously. This allows optimal electrochemical detection for the different components in the sample, thus increasing the sensitivity of the analysis. This method provides additional ability to identify substances because one has not only the optimal voltage for a particular compound but the ratios of peak areas or heights at different voltages, which can also be used as an identification tool. These methods have been developed at BASi for the Analytical Core of the Purdue University Botanical Center on Age Related Diseases.
Measurement of Tea Polyphenols in Different Teas
Teas:
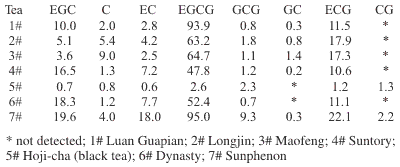
Green teas from China (Guapian, Longjin, and Maofeng), green teas from Japan (Suntory and Dynasty), black tea from Japan (Hoji-cha), and green tea extract from Japan (Sunphenon).
Sample Preparation:
Aqueous extraction. 50 mg tea was steeped in 10 mL 80º C water for 10 min, ultrasonicated for 10 min and filtered through a 4.5 µm nylon filter.
Analytical Conditions
LCEC System: BASi 480e chromatograph with a multi-channel amperometric detector BASi epsilon™) and ChromGraph v2.00 software.
Electrode: Four 2mm glassy carbon working electrodes in a radial flow cell
Potential: +900, 800, 600, 400 mV vs. Ag/AgCl
Column: C8 5 µm column (150 x 4.6 mm)
Mobile phase: 20mM sodium monochloroacetate, pH 2.8, 12% acetonitrile (v/v)
Flow rate: 1.1 ml/min
Four Channel Liquid Chromatography with Electrochemical Detection
Tea Polyphenols
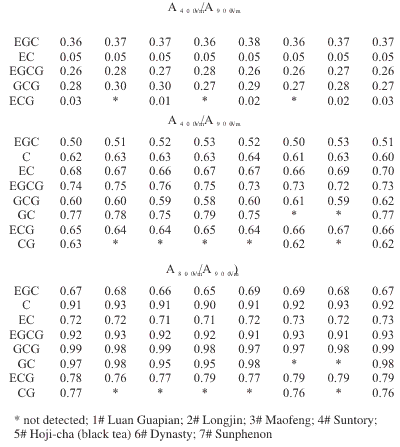
Ratios of Peak Area at Different Applied Potentials
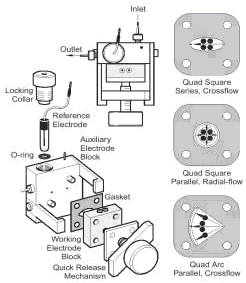
Different four-electrode cells which can be used with the Epsilon.
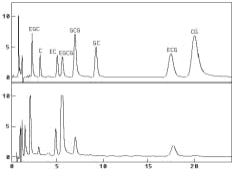
Chromatograms of standard mixture (A) and green tea extract (B) at +800 mV
Concentration of Polyphenols in Tea (mg/g)
In Vivo measurement of EGCg
Experimental Conditions
Sprague-Dawley Rats, 280-330 g, IV dose of EGCG 1 mg/kg. Blood sampled automatically using BASi Culex ® automated blood sampler.
Analytical Conditions
LCEC System: BASi 480e chromatograph with a multi-channel amperometric detector BASi epsilon™) and ChromGraph v2.00 software.
Electrode: Four 2mm glassy carbon working electrodes in a radial flow cell
Potential: +700, 600, 500, 400 mV vs. Ag/AgCl
Column: C8 5 µm column (150 x 4.6 mm)
Mobile phase: 20mM sodium monochloroacetate, pH 2.8, 12% acetonitrile (v/v)
Flow rate: 1.2 ml/min
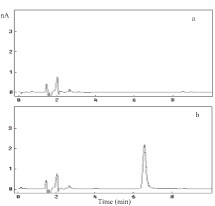
Chromatograms of (a) blank plasma and blood sample after i.v. administration of EGCg
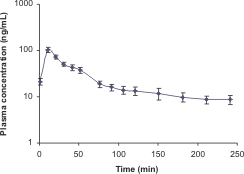
Mean (± S.D.) plasma concentration versus time profile of EGCG in rats (n = 4) following a single 2 mg/kg intraperitoneal administration
Identification of antioxidants from complex natural matrices
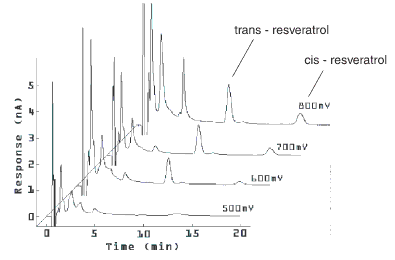
Resveratrol in wine
LCEC systems: PM-80 pump (BAS), ODS 3 mm, 100 x 2.0 mm column, a multi-channel amperometric detector BASi epsilon) coupled to four glassy carbon electrodes and referenced to an Ag/AgCl electrode
Chromatograms of red wine obtained from four-channel electrochemical detection.
Soy Isoflavones
Experimental Conditions
Gradient reverse-phase liquid chromatography with multi-channel electrochemical detectors set at +1100, 950, 850, 750 mV. Peak height ratios were used for peak identification.
Mobile phase A was composed of 9.3% acetonitrile, 5.9% methanol and 84.8% aqueous buffer (25 mM ammonium acetate, pH4.3, 0.25 mM EDTA). Mobile phase B was composed of 19.6 % acetonitrile. 12.0% methanol and 68.4% aqueous buffer (25 mM ammonium acetate, pH4.3, 0.25 mM EDTA). The gradient cycle was: 100% A for 1 min, 100% A to 80% A over 8 min, 80% A to 0% A over 1 min, 0% A for 10 min. 0% A to 100% A over 1 min, 100% A for 6 min. The flow rate was 0.6 mL/min.
Chromatograms of standard isoflavones and extract at potentials: +1100, 950, 850, 750 mV
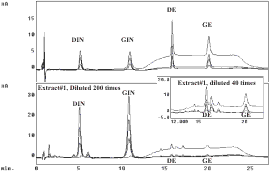
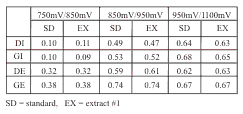
Comparison of peak height ratios for standards and retention equivalent peaks in Extract #1.